Hyperspectral imaging for early detection of Alzheimer’s disease
Amyloidopathic disorders such as Alzheimer’s disease present symptomology years following the entrenchment of amyloidogenic imbalance. Symptomology often presents only after significant neurodegeneration. There exists thus a warrant for early detection of amyloidopathy in Alzheimer’s disease. Non-existent modalities for direct identification and quantitation of soluble amyloid aggregates or (proto)fibrils forced us to undertake the development of a spectrophotometric technique to support ongoing drug design. Key requirements were independence from the need for extraneous staining, unambiguous amyloid aggregate detection and minimal influence of interpretative errors.
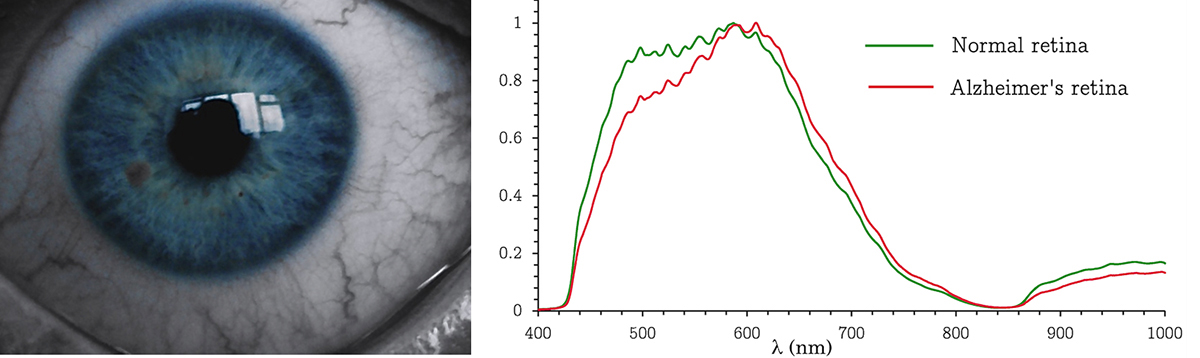
A Cytoviva® instrument pivotal to this study captures scattering of light of the visible–near infrared (VNIR, 400–1000 nm) wavelengths within each pixel of the microscopic view field. Assembled thusly was a scatterance intensity pattern database that lent “signatures” of amyloid aggregates.
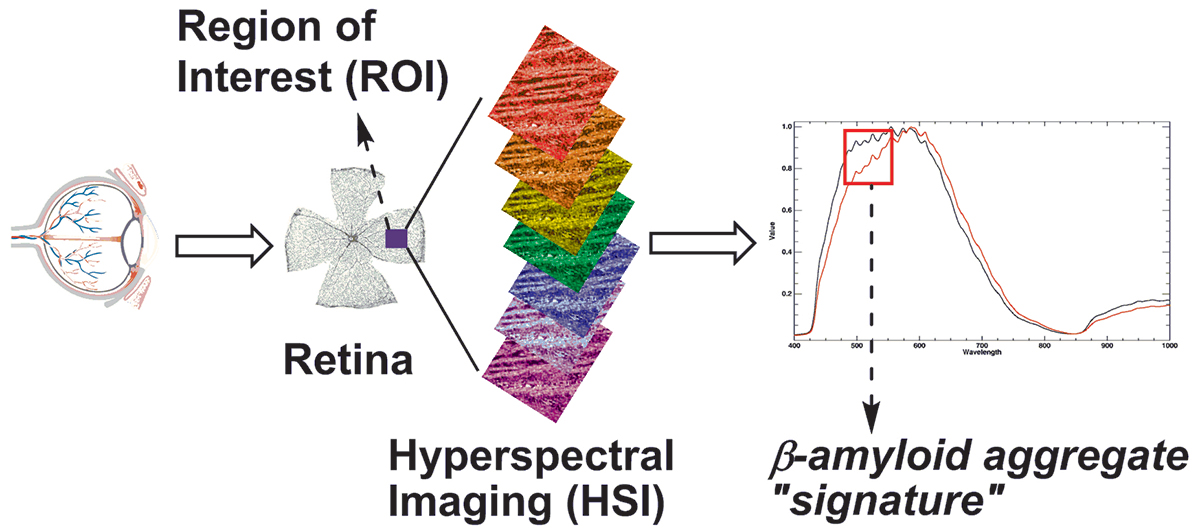
Comparison of unknown samples against this database enabled the direct detection of amyloid aggregates. The technique was found useful for monitoring retinal and brain amyloidopathy in an ongoing preclinical anti-AD study, attesting to the technique’s sensitivity and specificity. Interestingly, the technique was found applicable not just to excised brain tissue, but also to isolated mouse retina. With the retina being heralded widely as a (diagnostic) extension of the CNS and retinal amyloidopathy occurring well before that in the brain, this development raises a possibility for the first direct retinal imaging diagnosis of early, asymptomatic Alzheimer’s disease.
Diacetyl Prevents Cognitive Decline in Alzheimer’s Mouse Models
Diacetyl (DA), a food flavorant, is linked with occupational lung disease. Our in vitro experiments described the formation of a covalent adduct by DA with Arg5 of the Aβ1−42 peptide, which resulted in only a transient increase in neurotoxicity in SH-SY5Y cells. However, in vivo implications of these effects on Alzheimer’s disease (AD) pathogenesis and the underlying mechanisms remain poorly understood. In the APP/PS1 transgenic AD mouse model, DA treatment did not exacerbate learning and memory deficits in the Morris water maze test. Moreover, DA increased the Aβ1−42 plaque burden and decreased neuronal inflammation in the transgenic AD mice. Additionally, cognitive impairment induced by intracerebroventricular Aβ1−42 was restored by the DA treatment, as assessed by the T-maze test. A corresponding mitigation of neuronal inflammation was also observed in the hippocampus of these nontransgenic mice due to the acceleration of Aβ1−42 aggregation by DA into nontoxic plaques.
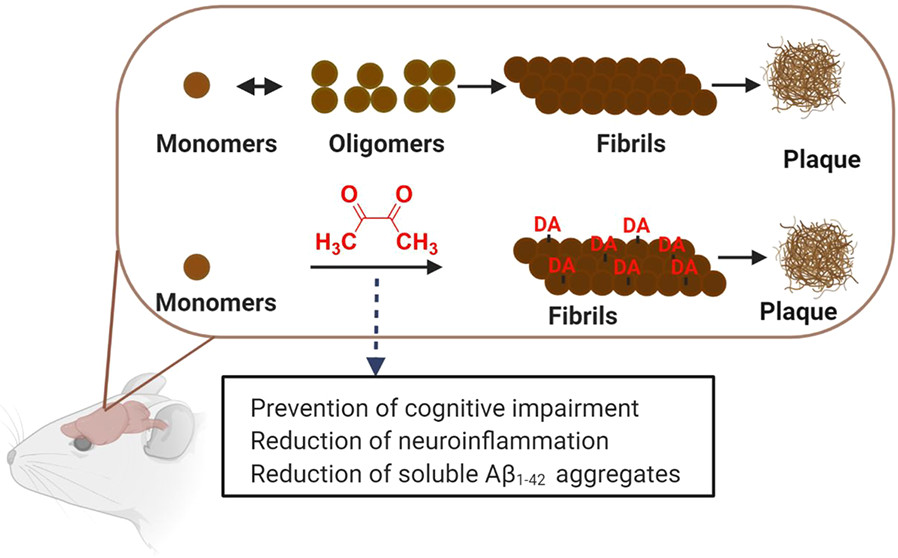
The data from SDS-PAGE, dot-blot, and TEM in vitro experiments corroborated the acceleration of the Aβ1−42 aggregation observed in vivo in AD animal models and characterized the DA-induced formation of Aβ1−42 fibrils. Such Aβ1−42−DA fibrils were unstable in the presence of detergent and amenable to detection by the thioflavin T reagent, thus underscoring the distinct assembly of these fibrils compared to that of the fibrils of the native Aβ1−42. Taken together, the results of this study present for the first time the in vivo implications of the DA-induced acceleration of Aβ1−42 and may provide a strategy for the rational design of Aβ1−42 aggregation accelerators as AD therapeutics that promote oligomer-free Aβ1−42 fibril formation.